

本文主要介紹了研究中實驗裝置的設計及測試的過程,主要包含半圓弧型器件微元腔體(ti) 器件。首先設計完成半圓弧器件,實現了把沉積過程和橢偏儀(yi) 測試相結合,觀察窗口選用石英玻璃,理論上zui大限度減小了光的損耗。
展示全部 
橢偏儀(yi) 在位表征電化學沉積的係統搭建(十九)- 圓形微流腔體(ti)
3.3微流腔體(ti)
由於(yu) 半弧形電解池存在的不足,所以進行了池體(ti) 的改進,得到圓形微腔體(ti) 以及進一步改進的流動長方形微腔體(ti) 。它們(men) 都有易於(yu) 拆卸更換電極、操作簡單等優(you) 點。
3.3.1圓形微腔
圖3-16是圓形微腔製作完成的實物圖以及在橢偏儀(yi) 測試中的放置示意圖。該池體(ti) 主要由基底即工作電極Au/Si、橡膠圈和上麵的打孔ITO即對電極組成,他們(men) 由上下亞(ya) 克力板即四角的螺絲(si) 固定壓緊。其中上麵的亞(ya) 克力板去掉了一塊留出觀察窗口,減小光在傳(chuan) 播過程中的損耗。ITO上打孔用於(yu) 溶液的注入。電極的連接由銅膠帶實現。
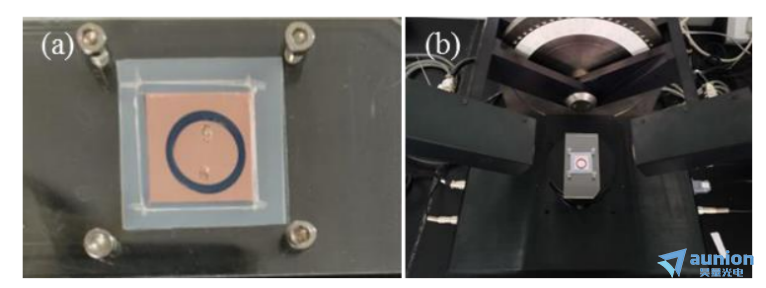
圖3-16(a)實物圖;(b)橢偏儀(yi) 測試中的放置圖
用該電解池進行了薄膜沉積,電解液為(wei) 0.02MCu(CH3COO)2,0.1MCH3COONa。由於(yu) 是兩(liang) 電極體(ti) 係Au/Si為(wei) 工作電極,ITO或Pb絲(si) 為(wei) 對電極,所以選擇恒壓沉積。由實驗組之前做的研究,選擇-0.4mA的電流進行沉積20分鍾。沉積結果如圖3-17所示,(a)為(wei) 以TIO作為(wei) 對電極沉積結果,(b)為(wei) 用Pt絲(si) 作(腔體(ti) 的上端由ITO換為(wei) 石英玻璃)為(wei) 對電極沉積的結果。
可以看到以ITO作為(wei) 對電極時,ITO上會(hui) 產(chan) 生大量的氣泡,而且實驗觀察可知氣泡會(hui) 隨著沉積時間的增加而變多變大。這樣以ITO為(wei) 對電極在該情況下進行橢偏測試不可行,因為(wei) 氣泡的存在會(hui) 極大影響光的傳(chuan) 輸,使得測試得到的數據基底的信息很難提取。
鑒於(yu) 沉積過程中會(hui) 在ITO上出現氣泡,所以考慮用Pb絲(si) 環代替ITO,如圖3-17(b)。可以看到在Pb絲(si) 環周圍同樣有氣泡產(chan) 生,但中間部分沒有,所以該方法改進了ITO做對電極的不足,用於(yu) 橢偏儀(yi) 測量可行。
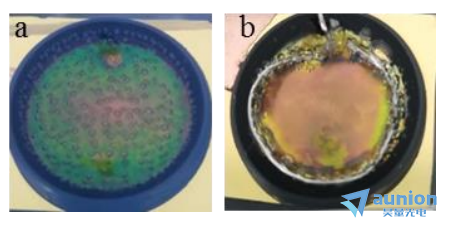
圖3-17不同對電極示意圖
上述圓形微腔體(ti) 改進是成功的,和圓弧形池體(ti) 對比它有電極更換容易、溶液層薄、密封性好等優(you) 點。但是該池體(ti) 也存在不足之處,如池體(ti) 氣泡始終存在、腔體(ti) 容納電解液少等。所以在該池體(ti) 基礎上進一步改進池體(ti) ,得到了長方體(ti) 流動微腔池體(ti) ,如下節詳述。
了解更多橢偏儀(yi) 詳情,請訪問上海昊量光電的官方網頁:
https://www.weilancj.com/three-level-56.html
更多詳情請聯係昊量光電/歡迎直接聯係昊量光電
關(guan) 於(yu) 昊量光電:
上海昊量光電設備有限国产黄色在线观看是光電国产欧美在线專(zhuan) 業(ye) 代理商,国产欧美在线包括各類激光器、光電調製器、光學測量設備、光學元件等,涉及国产成人在线观看免费网站涵蓋了材料加工、光通訊、生物醫療、科學研究、國防、量子光學、生物顯微、物聯傳(chuan) 感、激光製造等;可為(wei) 客戶提供完整的設備安裝,培訓,硬件開發,軟件開發,係統集成等服務。
您可以通過我們(men) 昊量光電的官方網站www.weilancj.com了解更多的国产欧美在线信息,或直接來電谘詢4006-888-532。
相關(guan) 文獻:
[1] WONG H S P, FRANK D J, SOLOMON P M et al. Nanoscale cmos[J]. Proceedings of the IEEE, 1999, 87(4): 537-570.
[2] LOSURDO M, HINGERL K. ellipsometry at the nanoscale[M]. Springer Heidelberg New York Dordrecht London. 2013.
[3] DYRE J C. Universal low-temperature ac conductivity of macroscopically disordered nonmetals[J]. Physical Review B, 1993, 48(17): 12511-12526. DOI:10.1103/PhysRevB.48.12511.
[4] CHEN S, KÜHNE P, STANISHEV V et al. On the anomalous optical conductivity dISPersion of electrically conducting polymers: Ultra-wide spectral range ellipsometry combined with a Drude-Lorentz model[J]. Journal of Materials Chemistry C, 2019, 7(15): 4350-4362.
[5] 陳籃,周岩. 膜厚度測量的橢偏儀(yi) 法原理分析[J]. 大學物理實驗, 1999, 12(3): 10-13.
[6] ZAPIEN J A, COLLINS R W, MESSIER R. Multichannel ellipsometer for real time spectroscopy of thin film deposition from 1.5 to 6.5 eV[J]. Review of Scientific Instruments, 2000, 71(9): 3451-3460.
[7] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[8] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[9] YUAN M, YUAN L, HU Z et al. In Situ Spectroscopic Ellipsometry for Thermochromic CsPbI3 Phase Evolution Portfolio[J]. Journal of Physical Chemistry C, 2020, 124(14): 8008-8014.
[10] 焦楊景.橢偏儀(yi) 在位表征電化學沉積的係統搭建.雲(yun) 南大學說是論文,2022.
[11] CANEPA M, MAIDECCHI G, TOCCAFONDI C et al. Spectroscopic ellipsometry of self assembLED monolayers: Interface effects. the case of phenyl selenide SAMs on gold[J]. Physical Chemistry Chemical Physics, 2013, 15(27): 11559-11565. DOI:10.1039/c3cp51304a.
[12] FUJIWARA H, KONDO M, MATSUDA A. Interface-layer formation in microcrystalline Si:H growth on ZnO substrates studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Journal of Applied Physics, 2003, 93(5): 2400-2409.
[13] FUJIWARA H, TOYOSHIMA Y, KONDO M et al. Interface-layer formation mechanism in (formula presented) thin-film growth studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Physical Review B - Condensed Matter and Materials Physics, 1999, 60(19): 13598-13604.
[14] LEE W K, KO J S. Kinetic model for the simulation of hen egg white lysozyme adsorption at solid/water interface[J]. Korean Journal of Chemical Engineering, 2003, 20(3): 549-553.
[15] STAMATAKI K, PAPADAKIS V, EVEREST M A et al. Monitoring adsorption and sedimentation using evanescent-wave cavity ringdown ellipsometry[J]. Applied Optics, 2013, 52(5): 1086-1093.
[16] VIEGAS D, FERNANDES E, QUEIRÓS R et al. Adapting Bobbert-Vlieger model to spectroscopic ellipsometry of gold nanoparticles with bio-organic shells[J]. Biomedical Optics Express, 2017, 8(8): 3538.
[17] ARWIN H. Application of ellipsometry techniques to biological materials[J]. Thin Solid Films, 2011, 519(9): 2589-2592.
[18] ZIMMER A, VEYS-RENAUX D, BROCH L et al. In situ spectroelectrochemical ellipsometry using super continuum white laser: Study of the anodization of magnesium alloy [J]. Journal of Vacuum Science & Technology B, 2019, 37(6): 062911.
[19] ZANGOOIE S, BJORKLUND R, ARWIN H. Water Interaction with Thermally Oxidized Porous Silicon Layers[J]. Journal of The Electrochemical Society, 1997, 144(11): 4027-4035.
[20] KYUNG Y B, LEE S, OH H et al. Determination of the optical functions of various liquids by rotating compensator multichannel spectroscopic ellipsometry[J]. Bulletin of the Korean Chemical Society, 2005, 26(6): 947-951.
[21] OGIEGLO W, VAN DER WERF H, TEMPELMAN K et al. Erratum to ― n-Hexane induced swelling of thin PDMS films under non-equilibrium nanofiltration permeation conditions, resolved by spectroscopic ellipsometry‖ [J. Membr. Sci. 431 (2013), 233-243][J]. Journal of Membrane Science, 2013, 437: 312..
[22] BROCH L, JOHANN L, STEIN N et al. Real time in situ ellipsometric and gravimetric monitoring for electrochemistry experiments[J]. Review of Scientific Instruments, 2007, 78(6).
[23] BISIO F, PRATO M, BARBORINI E et al. Interaction of alkanethiols with nanoporous cluster-assembled Au films[J]. Langmuir, 2011, 27(13): 8371-8376.
[24] 李廣立. 氧化亞(ya) 銅薄膜的製備及其光電性能研究[D]. 西南交通大學, 2016.
[25] 董金礦. 氧化亞(ya) 銅薄膜的製備及其光催化性能的研究[D]. 安徽建築大學, 2014.
[26] 張楨. 氧化亞(ya) 銅薄膜的電化學製備及其光催化和光電性能的研究[D]. 上海交通大學材料科 學與(yu) 工程學院, 2013.
[27] DISSERTATION M. Cellulose Derivative and Lanthanide Complex Thin Film Cellulose Derivative and Lanthanide Complex Thin Film[J]. 2017.
[28] NIE J, YU X, HU D et al. Preparation and Properties of Cu2O/TiO2 heterojunction Nanocomposite for Rhodamine B Degradation under visible light[J]. ChemistrySelect, 2020, 5(27): 8118-8128.
[29] STRASSER P, GLIECH M, KUEHL S et al. Electrochemical processes on solid shaped nanoparticles with defined facets[J]. Chemical Society Reviews, 2018, 47(3): 715-735.
[30] XU Z, CHEN Y, ZHANG Z et al. Progress of research on underpotential deposition——I. Theory of underpotential deposition[J]. Wuli Huaxue Xuebao/ Acta Physico - Chimica Sinica, 2015, 31(7): 1219-1230.
[31] PANGAROV n. Thermodynamics of electrochemical phase formation and underpotential metal deposition[J]. Electrochimica Acta, 1983, 28(6): 763-775.
[32] KAYASTH S. ELECTRODEPOSITION STUDIES OF RARE EARTHS[J]. Methods in Geochemistry and Geophysics, 1972, 6(C): 5-13.
[33] KONDO T, TAKAKUSAGI S, UOSAKI K. Stability of underpotentially deposited Ag layers on a Au(1 1 1) surface studied by surface X-ray scattering[J]. Electrochemistry Communications, 2009, 11(4): 804-807.
[34] GASPAROTTO L H S, BORISENKO N, BOCCHI N et al. In situ STM investigation of the lithium underpotential deposition on Au(111) in the air- and water-stable ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide[J]. Physical Chemistry Chemical Physics, 2009, 11(47): 11140-11145.
[35] SARABIA F J, CLIMENT V, FELIU J M. Underpotential deposition of Nickel on platinum single crystal electrodes[J]. Journal of Electroanalytical Chemistry, 2018, 819(V): 391-400.
[36] BARD A J, FAULKNER L R, SWAIN E et al. Fundamentals and Applications[M]. John Wiley & Sons, Inc, 2001.
[37] SCHWEINER F, MAIN J, FELDMAIER M et al. Impact of the valence band structure of Cu2O on excitonic spectra[J]. Physical Review B, 2016, 93(19): 1-16.
[38] XIONG L, HUANG S, YANG X et al. P-Type and n-type Cu2O semiconductor thin films: Controllable preparation by simple solvothermal method and photoelectrochemical properties[J]. Electrochimica Acta, 2011, 56(6): 2735-2739.
[39] KAZIMIERCZUK T, FRÖHLICH D, SCHEEL S et al. Giant Rydberg excitons in the copper oxide Cu2O[J]. Nature, 2014, 514(7522): 343-347.
[40] RAEBIGER H, LANY S, ZUNGER A. Origins of the p-type nature and cation deficiency in Cu2 O and related materials[J]. Physical Review B - Condensed Matter and Materials Physics, 2007, 76(4): 1-5.
[41] 舒雲(yun) . Cu2O薄膜的電化學製備及其光電化學性能的研究[D]. 雲(yun) 南大學物理與(yu) 天文學院,2019.
展示全部 